Monitoring Cyanobacteria in Grahamstown Dam
V Shah, D Turner, C Hancock, P O’Donoghue, A Sneddon, J Stanmore, A Morrow, A Lundmark, J Bates, A Hanson.
First published in Water e-Journal Vol 5 No 4 2020.
Introduction
Grahamstown Dam (capacity about 182,000 ML), Hunter Water’s largest dam, is a broad, relatively shallow, man-made, off-river storage that is primarily used to store water extracted from the Williams River. The Dam also receives runoff from its own small 73 km2 catchment area and direct rainfall on its 28 km2 surface area.
The key components of the Grahamstown Dam supply scheme are Seaham Weir (limits the upstream movement of tidal saltwater), Balickera Canal and pumping station (transfer water from the Williams River to Grahamstown Dam), Campvale Pumping Station (pumps run off from the developing Medowie area located on the eastern margins of the Dam), George Schroder Pumping Station and delivery mains (delivers water from the Dam to water treatment plant), and Grahamstown Water Treatment Plant (WTP).
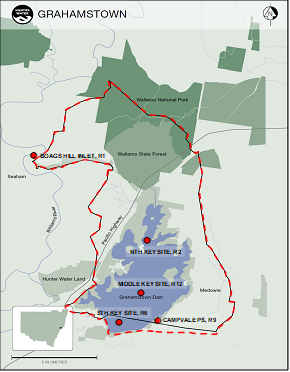
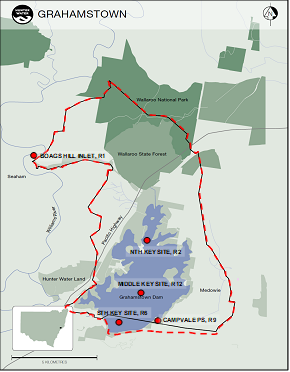
Routine algae monitoring locations within Grahamstown Dam catchment are the Dam’s key northern (R2), middle (R12) and southern (R6) locations, Campvale Pump Station (R9) and Boags Hill at Seaham Weir (R1) (Figure 1). The raw water offtake is located near the Dam’s key southern monitoring location R6. To protect drinking water quality, public access to Grahamstown Dam is minimised for recreational purposes.
Methodology
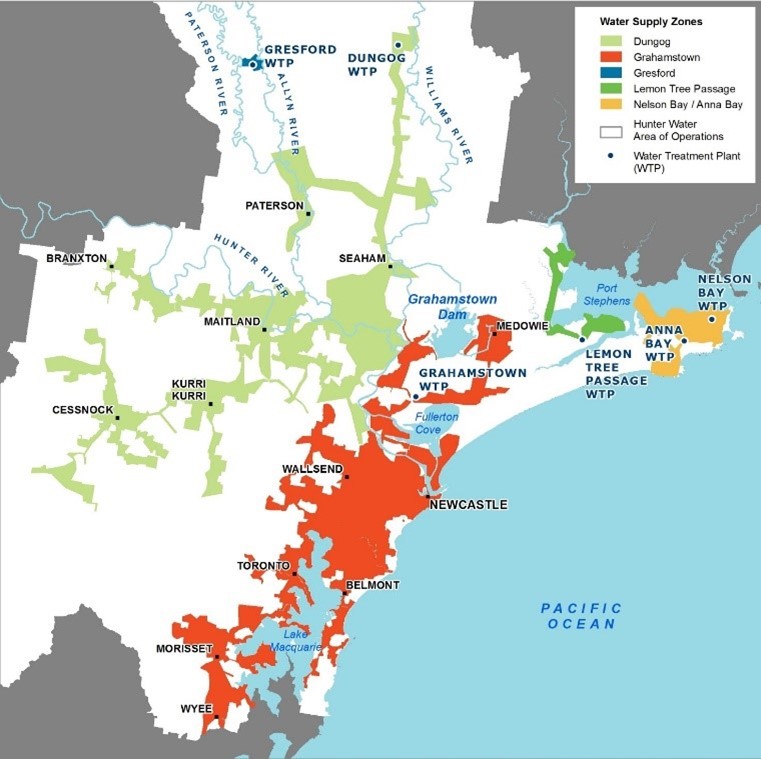
Grahamstown Dam supplies raw water to Grahamstown WTP, Hunter Water’s largest WTP, to supply drinking water to a population of 400,000 people in the Lower Hunter Region of NSW (Figure 2). Grahamstown WTP uses conventional treatment processes, which includes coagulation/flocculation, sedimentation, filtration, pH correction, disinfection and fluoridation. A Powdered Activated Carbon (PAC) dosing facility is located at Grahamstown Dam immediately downstream of the raw water offtake and five kilometres north of Grahamstown WTP. This Dam and associated WTP are critically important to Hunter Water as it is not possible to meet customer demand outside of winter without extraction from this source, which can meet up to 75% of Hunter Water’s water supply requirements on a high-usage day.
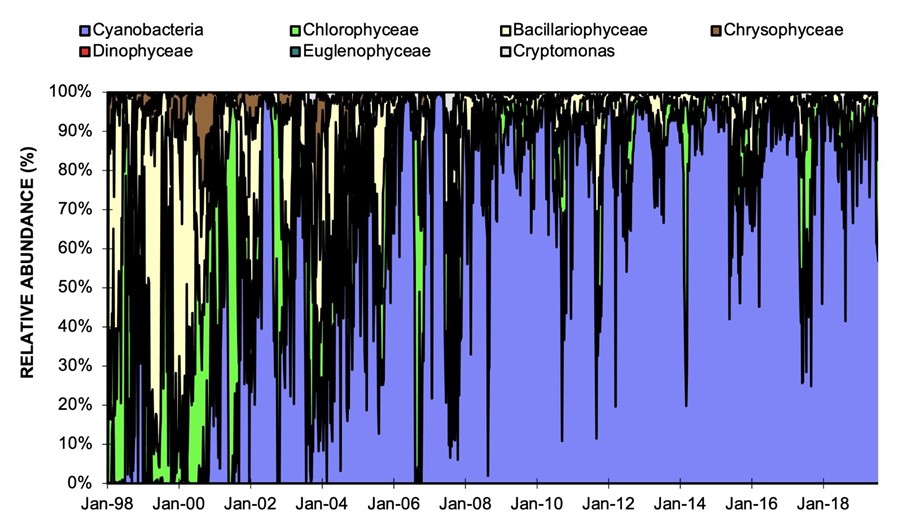
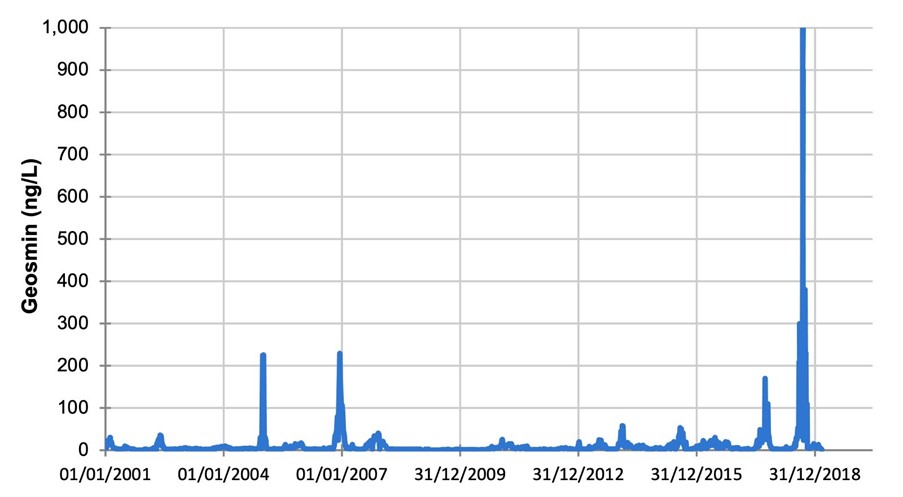
Cyanobacteria can produce objectionable taste and odour compounds, such as geosmin, and toxins that can pose potential health hazards to freshwater systems and supply of safe drinking water. Historical water quality monitoring data show a gradual increase in relative abundance of cyanobacteria in Grahamstown raw water (Figure 3) with occasional spikes in Dolichospermum and Microcystis levels for short periods without resulting in a major algae bloom.
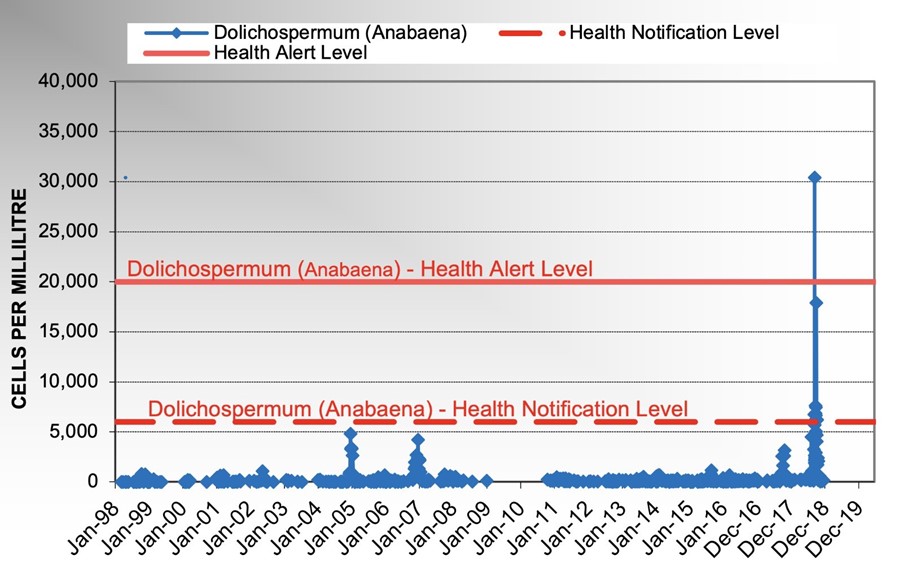
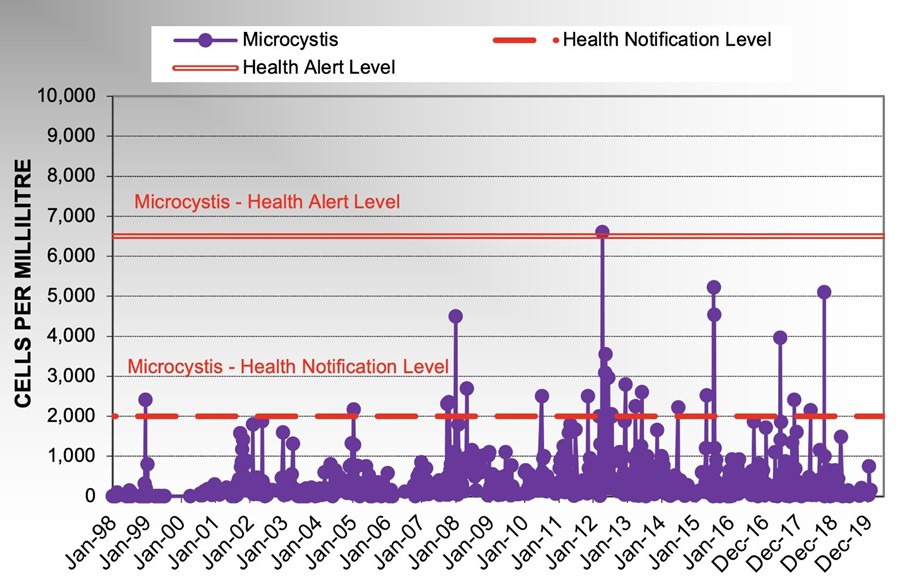
The highest counts for Dolichospermum and Microcystis recorded in the Grahamstown raw water prior to this incident were 4800 cells/mL in December 2004 and 6600 cells/mL in December 2012, respectively (Figure 4 and Figure 5).
Figure 5: Historical Microcystis Levels in Grahamstown Raw WaterIn 2011, Hunter Water conducted an Adaptive Management Strategy to better characterise the ecology of Grahamstown Dam for factors which could lead to, or influence, the development of harmful algae blooms.As part of this study, a predictive Bayesian Network Model of Algae Growth in Grahamstown Dam was developed which provided predictions of extremely low likelihood of moderate to severe algae blooms occurring in Grahamstown Dam.Groundwater from Hunter Water’s Tomago Borefield is an important water supply when Grahamstown source is compromised by algae blooms and also during drought. In order to use the water supply effectively, Hunter Water needs to be able to activate this source at short notice, when the need arises.
Results and Discussion
Largest cyanobacteria bloom in Grahamstown Damon record
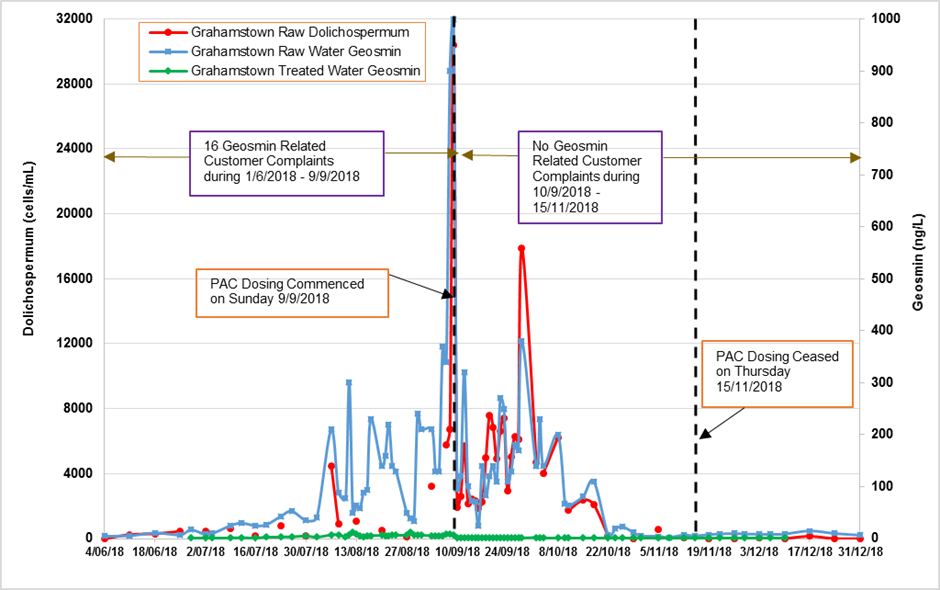
Geosmin levels in Grahamstown raw water started to rise
notably in the first week of September. On 5 September 2018, Hunter Water rangers reported an algae scum near the spillway in the northern part of Grahamstown Dam (Figure 7). No algae scums were observed the next day. Wind direction in the dam impacted positioning of algae scums and, in turn, the measured algae levels in raw water supplied to Grahamstown WTP. Cyanobacteria results from the dam and raw water samples collected earlier in the week were well below Australian Drinking Water Guidelines (ADWG, 2011)notifications levels(cells/mL)for Dolichospermumcircinale(6,000) and Microcystis aeruginosa(2,000).
On 7 September 2018, Hunter Water’s rangers reported algae scums with a green tinge on the southern shoreline of the Dam near the raw water off take. Grahamstown raw and treated water samples,along with algae scum samples were collected for algae and geosmin analysis.Raw water Dolichospermumlevels were still below ADWG notification levels with geosmin levels in treated water <10 ng/L. The algae scum collected from Grahamstown Dam southern shore line reported 280,000 Dolichospermumcells/mL. Given the close proximity of algae scums to the raw water off take area, Hunter Water’s treatment operations contractor, Veolia, was notified on 7 September to ensure that the PAC plant was ready for operation to remove extracellular geosmin if required at short notice. On 8 September, algae monitoring in Grahamstown Dam and WTP continued with raw water Dolichospermumlevels of 6750 cells/mL, which exceeded the health notification level of 6000 cells/mL. Elevated levels of Dolichospermum circinale(up to 30,400 cells/mL) and geosmin (up to 1000 ng/L) were detected in raw water samples collected on 9 September (Figure 8). On 9 September 2018, Hunter Water notified NSW Health of these results and commenced PAC dosing. Hunter Water declared a Major Incident, and an Incident Management Team was established. Public access to the dam for recreational activities was ceased, and customers extracting untreated dam water were advised of the issue and told to refrain from using it for irrigation and livestock. Leading up to 8 September, monitoring was being undertaken as per Hunter Water’s Cyanobacterial Framework for Potable and Environmental Waters and, in addition, more frequent monitoring of Grahamstown Dam and WTP locations was also carried out as necessary. Water NSW was also notified of these results as part of Hunter Water’s Cyanobacterial Framework for Environmental Waters.
Intense catchment surveillance and monitoring for cyanobacteria, toxins and geosmin
From 7 September 2018, Hunter Water commenced intense surveillance of the Dam with daily reports on observations. More intense sampling and analysis of raw and treated water for geosmin, cyanobacteria and toxins also commenced. From 8 September onwards, daily monitoring of Grahamstown Dam and WTP locations were taking place until 13 September, gathering data to guide the initial incident response. On 12 September, a formal algae monitoring plan was developed, based on the available results, to assist in responding to this incident. Samples for extracellular and intracellular geosmin and PhytoxigeneTM testing were sent to Sydney Water Laboratory in Sydney. Samples for algae toxins testing were sent to Symbio laboratory in Brisbane. Total geosmin, algae counts, nutrient analysis as well as other routine testing and sampling for this incident was carried out by the Australian Laboratory Services (ALS) laboratory in Newcastle.
An auto sampler was installed upstream of the PAC dosing location on 14 September to gather data on diurnal patterns in an attempt to inform optimisation of PAC dosing and raw water extraction. Diurnal monitoring of Grahamstown raw water (pre-PAC location at Schroder Pump Station) did not appear to provide useful data for the incident response and did not correspond with the raw water results at Grahamstown WTP. Monitoring of algae at different depths in Grahamstown Dam locations did not appear to provide consistent trends or useful data for the incident management. The high results observed in algae scums and water samples collected near the raw water offtake, and Middle of Dam (R12) locations were not realised in raw water at Grahamstown WTP.
A total of 46 algae scum and water samples collected from Grahamstown Dam (three routine key monitoring locations and Schroder Pump Station at raw water offtake area) and Grahamstown WTP raw water, backwash recovery and treated water were analysed for algae toxins (Anatoxin-a, Cylindrospermopsin, Deoxycylindrospemopsin, Microcystin-RR, Microcystin-YR, Microcystin-LR, Microcystin-Total, Nodularin, Saxitoxin, Neosaxitoxin, Saxitoxin GTX2 & 3, Saxitoxin GTX1 & 4, Saxitoxin C1 & C2, and Saxitoxin dcGTX2&3) throughout the incident. No toxins were detected in any of these samples. Potential for productions of Cylindrospermopsin gene cyrA and Saxitoxin gene sxtA was not detected by PhytoxigeneTM in any of the 10 samples analysed (including the ‘worst’ sample i.e. highest algae counts for algae scum sample during this incident). However, 2200 gene copies/mL of Microcystin/Nodularin gene mcyE were detected in the ‘worst’ algae scum sample collected from southern shoreline of dam. This sample had Dolichospermum and Microcystis counts (cells/mL) of 2,903,700 and 6500 respectively. This sample was also analysed for algae toxins but none were detected. Together with decreasing algae levels in the raw water, this indicated a low likelihood of toxin production for the remainder of the incident. Hunter Water then adopted the approach of conducting algae toxin testing on an ad-hoc basis in any samples over the ADWG health notification and alert levels and from any algae scums observed.
Sampling was undertaken on the spent backwash return to assess whether there was a risk for recirculation and concentration of Dolichospermum or Microcystis. There were no detects for Dolichospermum or Microcytis, indicating that the gravity settling process was effective in removing the algae cells. Some non-toxigenic algae cells, such as Planktolyngbya and Pseudanabaena, were detected in the spent backwash return and also in treated waters (in very small numbers) which indicated these small celled algae were difficult to remove through gravity settling, but were mostly removed through filtration. These algae cells do not produce taste and odour compounds or toxins. The issue was managed through periodic purging of spent backwash return to the sludge lagoon, which was not recycled.
Optimisation of water treatment processes
A desktop study comparing different types of PAC for geosmin removal was conducted. Previous jar testing investigations undertaken on Grahamstown Dam waters were useful in initially determining the PAC dose. The PAC dosing rate was initially adjusted on 12th September in accordance with WQRA / CRC CARE report #74 Management Strategies for Cyanobacteria (blue-green algae): A Guide for Water Utilities (Table 19, page 69).
The treatment processes were continually optimised based on the available monitoring results. Intracellular geosmin (~80%) was largely removed by conventional treatment processes and extracellular geosmin was adsorbed by PAC. Optimised treatment processes included a low PAC dose rate of 10 mg/L with a contact time of between 80 and 290 minutes, coagulation pH 6-6.5, increased alum dose, filters operated to maintain filtered turbidity <0.15 NTU, and chlorine at treated water tank inlet raised from 2.3 mg/L to 3 mg/L with chlorine exiting the plant 2.5 mg/L and Ct in excess of 100 min.mg/L. Filter head loss and run time for change were monitored. The backwash recycle stream was diverted to waste to reduce the risk of cyanobacteria being recirculated in the process.
Incident management
Hunter Water’s approach to incident management involved the development of plans based on the most likely and worst-case outcome. Due to the unprecedented scale of the algae event and uncertainty regarding whether toxins would be produced, management of the incident was very challenging.
On 12 September, the incident was further discussed with relevant stakeholders at Hunter Water’s Water Quality Committee meeting. In this meeting, disccusions covered site observations especially on the presence and positioning of algae scums in the dam, review of the available algae and geosmin results in the dam, raw and treated water, the status of current treatment processes, the status of customer complaints, the need for optimisation of sampling program and treatment processes including types of PAC and dosing rates. The minutes of the meeting were sent to NSW Health to update them regarding the current status of the incident. Notable follow up actions assigned to nominated Hunter Water staff and Hunter Water’s contractors Veolia and ALS included optimisation of treatment processes including using the most effective PAC type and dosing rate, and adjusting chlorine dose, implementing the revised water quality monitoring plan, liaising with NSW Health, and the effective management of the incident.
Source substitution using the Tomago Borefields was also considered given the potential for high risk of algae in surface water extracted from Grahamstown Dam. Switching to the groundwater source carried its own risks associated with a reduction in water supply capacity and potential issues associated with elevated levels of manganese in treated waters and therefore this option was not implemented.
On 17 September, a workshop was organised with the Australian Water Quality Centre (AWQC) to discuss Hunter Water’s incident response and seek their advice. During the workshop, it was found that that Hunter Water’s response to this algae event that included a monitoring plan to characterise the event, review of data and ongoing optimisation of treatment processes was generally adequate. The AWQC provided timely advice on adequacy of the sampling program, robustness of optimisation of treatment processes and algae toxin testing.
On 24 September, a stakeholder meeting was organised to discuss Hunter Water’s ongoing response to recent and other potential scenarios of algae and geosmin detections, and accordingly the monitoring plan was further modified from 1 October. Grahamstown WTP operations were continually optimised based on changes reflected by algae and geosmin results including whether to recycle or not recycle backwash water back to the WTP, and adjusting PAC, alum and chlorine dosing rates.
The continued non-detection of algae toxins meant this incident was ultimately classified as an aesthetic issue. Leading up to the incident, geosmin levels in Grahamstown treated water were elevated with two spikes just over 10 ng/L, which resulted in occasional customer complaints. Once the event unfolded there was no increase in customer complaints, showing that the operational response including the PAC dosing was effective in addressing the aesthetic issue.
Conclusions
Algae toxins were not detected throughout the incident in any of the samples collected from Grahamstown Dam, raw and treated water locations. This incident was ultimately classified as a taste and odour issue. No geosmin-related taste and odour customer complaints were received after the PAC dosing was commenced, and treatment processes at Grahamstown WTP were continually optimised based on the review the intense water quality monitoring data. Thus, Hunter Water’s operational response was quite effective in addressing the aesthetic issue during the largest algae bloom on record in Grahamstown Dam.
Key observations and learnings
- The persistence presence of geosmin in Grahamstown raw water for an extended period during June – August 2018 led to the onset of an unprecedented algae bloom that unfolded in September 2018. This observation could be used to predict future algae blooms.
- Wind direction in the dam impacted positioning of algae scums and hence the Dolichospermum levels in Grahamstown raw water. While extremely high algae results were observed in algae scums and Grahamstown Dam locations, these were generally not observed in raw water supplied to Grahamstown WTP. This identified the potential benefits of continually observing wind directions during future algae blooms.
- Diurnal monitoring of raw water and depth profiling of dam water did not provide useful algae data for the management of this incident. Data obtained from this monitoring did not provide a consistent trend to make meaningful use of these data. This monitoring may not be useful during future events.
- Hunter Water’s cyanobacterial contingency plans have been revised to include more detailed guidance on monitoring and operational response for an algae toxin event to maximise safe and reliable treated water in the case of a future severe algae event.
- Potential issues with reliability and capability of the PAC facility to manage an algae toxin event were identified, and are being addressed.
- The previous prediction of low chances of a moderate to severe algae bloom occurring in Grahamstown Dam was revisited and after including the data for this unprecedented algae bloom, the new prediction for probability of a moderate algae bloom in Grahamstown Dam has been now revised to about once in 10 years.
KEY FUTURE INITIATIVES
A number of vulnerabilities were identified during the operational response to this unprecedented algae bloom in Grahamstown Dam. Hunter Water is currently working on the following key initiatives to better prepare for future algae blooms:
- Hunter Water is currently assessing options for additional treatment barriers at Grahamstown WTP for a severe algae bloom with presence of algae toxins. The replacement of Stage 2 filters at Grahamstown WTP is required due to an asset condition issue. A business case to replace the current media with biologically activated carbon is being prepared. This will provide an enhanced barrier to saxitoxin. Options for advanced oxidation on the current process will be investigated as both an additional barrier to saxitoxin and to some emerging contaminants. An engagement is currently underway assessing the effectiveness of the existing barriers (PAC, filtration and chlorination) to saxitoxin. This assessment is utilising the Water RA CRAST tool https://www.waterra.com.au/project-details/168.
- Rapid toxin test kits are being trialled to assess their reliability and accuracy. Hunter Water has undertaken some limited trials to date, both in conjunction with the University of Newcastle (in an undergraduate teaching laboratory) and in the field during the Microcystis bloom in Chichester Dam in 2020 using the BlueGreenTest®, a field-based kit that can be used for the rapid detection of some cyanotoxins (microcystins) in water. Based on the very limited trials Hunter Water has done to date, the kits seemed to show some promise in detecting toxins at concentrations below the ADWG guideline value for microcystin, indicating that this could be a valuable addition to more traditional toxin testing methods.
- CyanoLakes remote sensing system is being trialled in Grahamstown Dam to assess its future usefulness for providing an early warning of an algae bloom. CyanoLakes is an online monitoring and mapping service for detecting trophic status and cyanobacterial presence in inland waters using satellite remote sensing. Using a Maximum Peak Height (MPH) algorithm, it can detect and map optically active pigments, including chlorophyll-a and phycocyanin, which represent proxies for phytoplankton biomass and cyanobacterial presence respectively. From a preliminary study based on limited data, CyanoLakes seemed to provide an earlier detection of bloom development, and give a more extensive spatiotemporal view of the algal bloom dynamics than that obtained via traditional grab sampling and analysis. Future work is currently being done to assess the accuracy of this service via a ground-truthing experiment and look into the use of spatial satellite imagery for long-term trend analysis.
About the authors
Vikas Shah | As Treatment Engineer at Hunter Water, his role is to interface with Hunter Water’s treatment operations and maintenance and laboratory services contractors to contribute to the management and development of systems that facilitate the supply of continuously safe drinking water.
Dave Turner | Dave is a chemical engineer and manages the Water Network Operations team for Hunter Water. He has 20 years’ experience in the water industry focussing on water quality in the treatment and network areas.
Pam O'Donoghue | Pam O’Donoghue has 36 years of experience in the water industry. As Water Treatment Operations Engineer at Hunter Water, her role is to interface with Hunter Water's Treatment operations and maintenance contractor and contribute to the management and development of systems that facilitate the supply of continuously safe drinking water.
Ashley Sneddon | As Manager Water Treatment Operations at Hunter Water he is responsible for interfacing with Hunter Water's Treatment Plant operation and maintenance contractor to ensure the ongoing support of safe, compliant, effective and efficient supply of potable water from Hunter Water's 6 water treatment plants.
Abigail Morrow | As a water quality scientist in Hunter Water’s Science and Innovation team, Abigail Morrow provides scientific advice across the organisation on nuisance and harmful algae, emerging contaminants and other aspects of water quality relevant to public and environmental health, and supports research and innovation activities, strategy development and evidence-based decision-making.
Anna Lundmark | Anna Lundmark is the Manager for Science and Innovation at Hunter Water, a team responsible for the R&D and innovation strategies, and the provision of scientific expertise in the areas of water, waste water, recycled water, and biosolids. Anna is a certified CEnvP Site Contamination Specialist.
Jordi Bates | Jordi Bates is a senior water engineer and team leader with 20 years work experience in the public and private sectors in Australia and overseas. He is currently team leader in the Water Resilience strategic planning team at Hunter Water, NSW, working on long term water security planning for the Lower Hunter region.
Axel Hanson | Experienced Catchment Scientist and Engineer working in Hunter Water’s Service Delivery For Customers Operations team. Axel Hanson specialises in environmental water quality, quantity and infrastructure projects, including monitoring and reporting on drinking water catchments, dams, aquifers and wastewater network.
Colin Hancock | Colin is the Group Manager of the Water Operations group. The group is responsible for the provision of a safe and reliable water supply to the lower Hunter community.
John Stanmore | Technical Lead (Water Supply Systems)
References
NHMRC (2011) Australian Drinking Water Guidelines https://www.nhmrc.gov.au/about-us/publications/australian-drinking-water-guidelines
WQRA / CRC CARE (2010) Management Strategies for Cyanobacteria (blue-green algae): A Guide for Water Utilities Report #74 (Table 19, page 69).